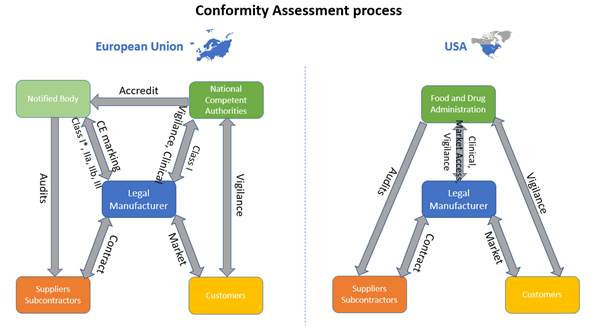
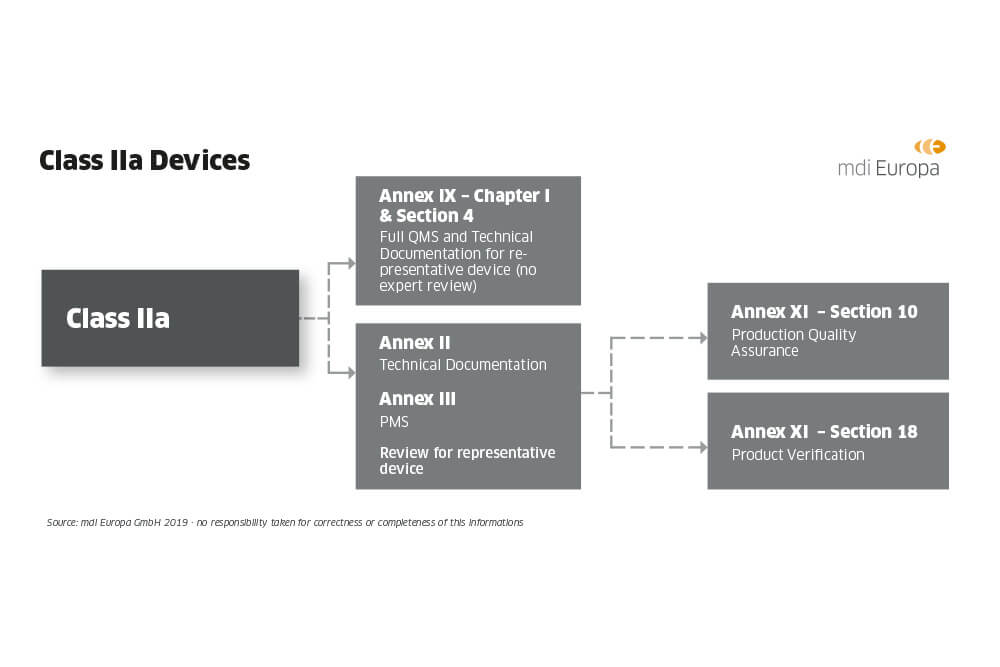
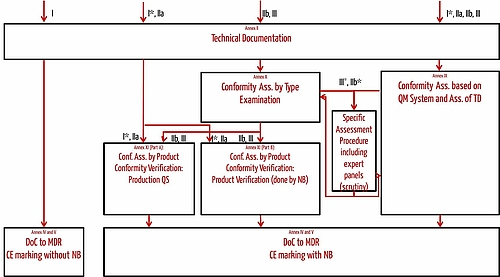
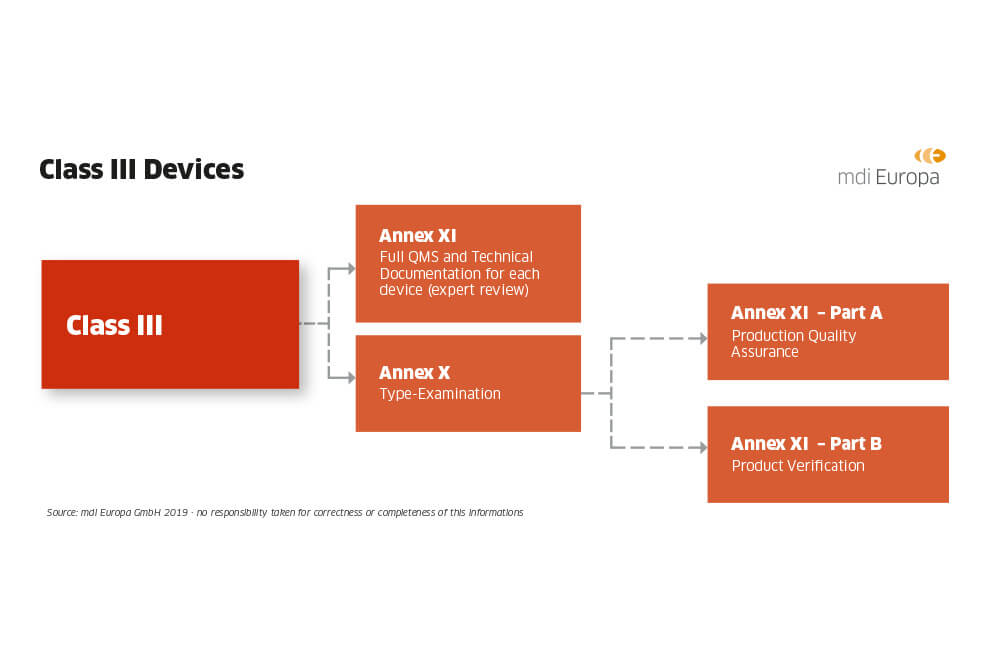
How are medical devices regulated in the European Union? - Elaine French-Mowat, Joanne Burnett, 2012
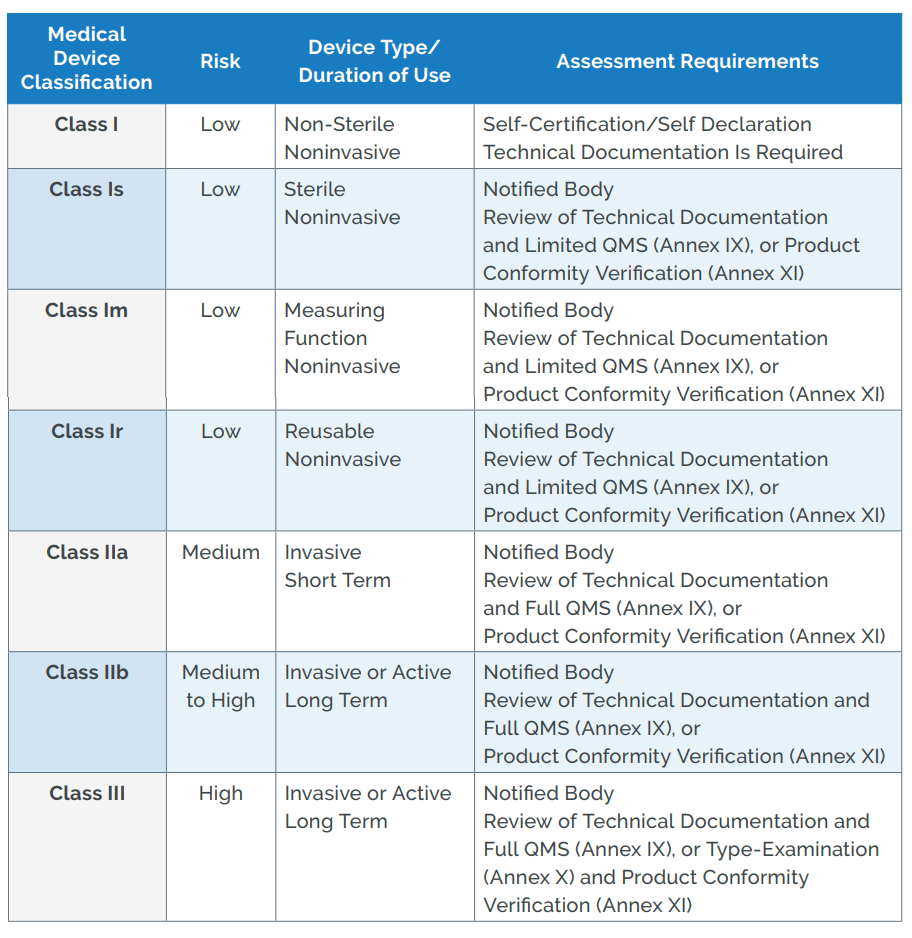
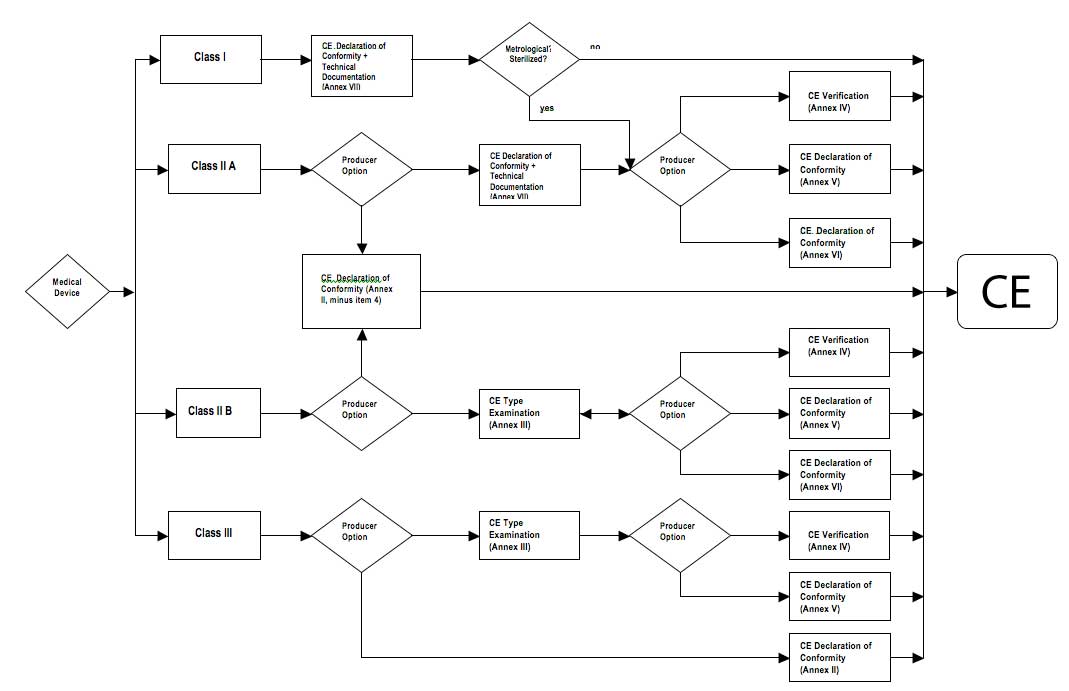
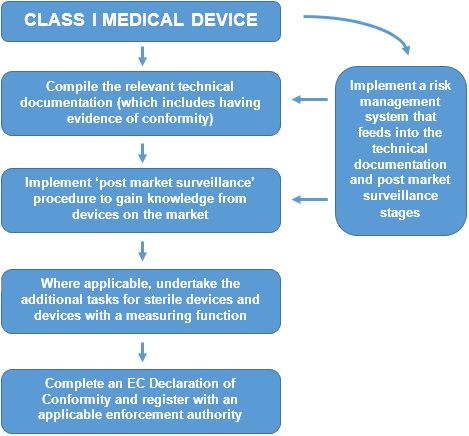
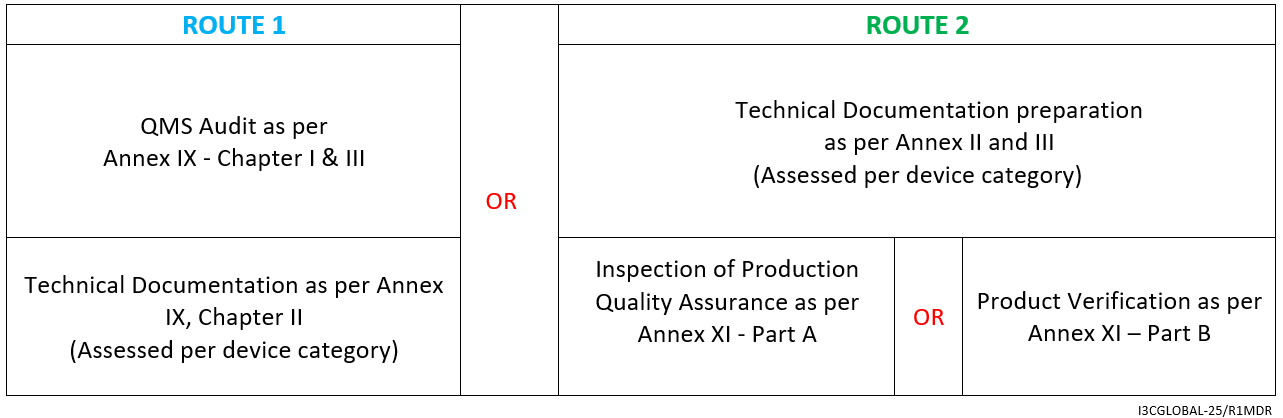
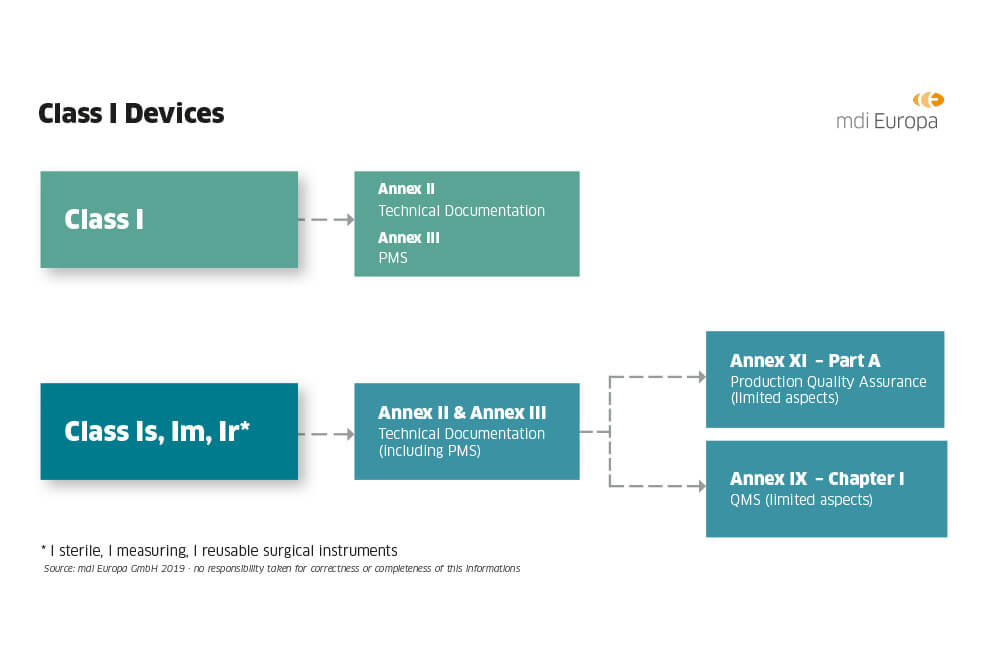
Guide on Class I (Is/Im) MDD- Medical Devices CE marking (mark) & European (EU) Authorized Representative service
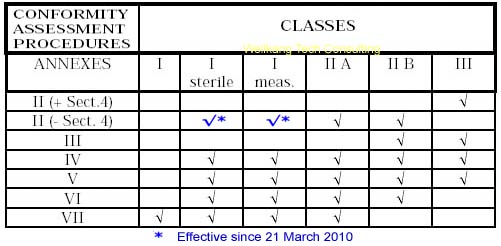
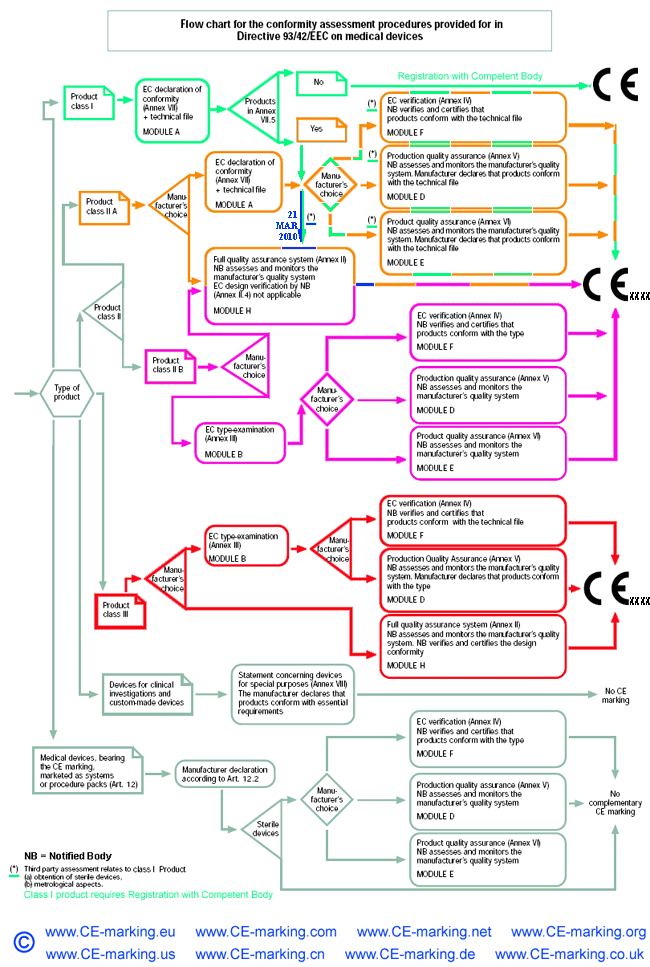
Guide on Class I (Is/Im) MDD- Medical Devices CE marking (mark) & European (EU) Authorized Representative service